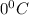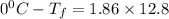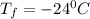Answer: The freezing point of solution is
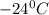
Explanation:
Formula used for elevation in boiling point is,
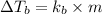
where,
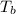 = change in boiling point
= change in boiling point
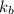 = boiling point constant=
= boiling point constant=
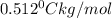
m = molality
Given: Boiling point of solution =
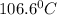
Boiling point of water=
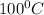
thus
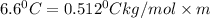
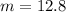
Formula used for lowering in freezing point is,
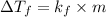
where,
 = change in freezing point
= change in freezing point
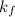 = freezing point constant =
= freezing point constant =
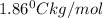
m = molality
Freezing point of water=