Answer:
The final freezing point of water is -5.58
Step-by-step explanation:
According to colligative properties of molecules,
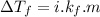
where
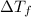 is the depression in freezing point of a solution, i is the vant hoff factor of solute,
is the depression in freezing point of a solution, i is the vant hoff factor of solute,
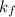 is the cryogenoscopic constant of solvent and m is molality of the solution.
is the cryogenoscopic constant of solvent and m is molality of the solution.
Here solute is sugar and solvent is water.
Molality of solution =
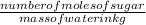 =
=
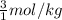 = 3 mol/kg
= 3 mol/kg
So
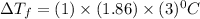 = -5.58
= -5.58