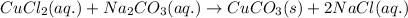Answer:
The balanced chemical reaction is -
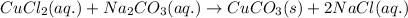
Step-by-step explanation:
According to solubility rule,
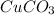 is an insoluble compound and NaCl is a water soluble compound.
is an insoluble compound and NaCl is a water soluble compound.
The given precipitation reaction involves exchange of cations and anions between
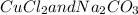 , resulting formation of insoluble
, resulting formation of insoluble
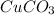 and NaCl.
and NaCl.
Balanced equation: