Answer:- Molar mass is 92.59 gram per mol.
Solution:- Depression in freezing point is directly proportional to the molality of the solution. The equation we use for this type of problems is:
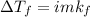
where,
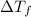 is depression in freezing point, i is Van't hoff factor, m is molality and kf is the freezing point depression constant.
is depression in freezing point, i is Van't hoff factor, m is molality and kf is the freezing point depression constant.
Toluene is a non electrolyte and so the value of i for this is 1.
depression in freezing point is given as 0.47 and the kf is given as 5.12. So, we could calculate the molality of the solution using the equation written above.
Let's plug in the values in the equation:
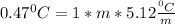
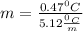
m = 0.0918 m
It means the molality of the solution is 0.0918 mol/kg.
We know that molality is, moles of solute per kg of the solvent. Mass of solvent is 100. g that is 0.100 kg. Now, we could calculate the moles of the solute that is toluene as:
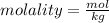
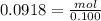
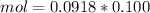
mol = 0.00918
Mass of solute is given as 0.85 g. We divide the grams by the moles to get the molar mass.
Molar mass =
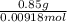
Molar mass =
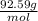
So, the molar mass of toluene is 92.59 gram per mol.