Answer: Because some of the processes are also known as incomplete combustion.
Step-by-step explanation:
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule. The chemical equation for this reaction follows:
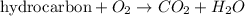
If supply of oxygen gas is limited, it is known as incomplete combustion and carbon monoxide gas is also produced as a product. The chemical equation for this reaction follows:
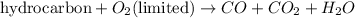
Thus, we cannot say that all the combustion reactions produce carbon dioxide and water as products because it depends on the supply of oxygen gas.