0.933 moles of ZnCl₂.
Step-by-step explanation
How many moles of Zn in 61.0 grams of zinc?
The relative atomic mass of zinc Zn is 65.38. As a result, the mass of one mole of Zn atoms is 65.38 grams.
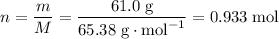
There are 61.0 / 65.38 = 0.933 moles of Zn atoms in 61.0 grams of Zn.
How many moles of ZnCl₂ can be produced from 0.933 moles of Zn?
Zn reacts with HCl by the equation:
Zn + 2 HCl → ZnCl₂. Balanced.
Each mole of Zn atoms combine with two moles of HCl to produce one mole formula unit of ZnCl₂. 0.933 moles of Zn atoms reacts with excess HCl to produce 0.933 moles formula units of ZnCl₂.