Answer:
N₂ gas at 50 °C.
Step-by-step explanation:
- From the postulates of the kinetic theory of gases; all gases at a given temperature have the same average kinetic energy.
- The average kinetic energy of gases can be calculated from the relation: K =
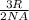 T.
T.
Where, K is the average kinetic energy (measured in Joule),
R is the general gas constant (R = 8.314 J/mol.K)
NA is Avogadros number (6.023 x 10²³ atoms/mol)
T is the temperature (measured in Kelvin).
- It is clear from the relation that K depends only on the temperature.
- So, N₂ gas at 50 °C has the highest average kinetic energy since it is at the higher temperature than other gases.