Answer : The correct option is, (b) 22.1 g
Solution : Given,
Mass of iron = 15.5 g
Molar mass of iron = 56 g/mole
Molar mass of
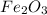 = 160 g/mole
= 160 g/mole
First we have to calculate the moles of iron.
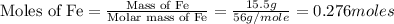
Now we have to calculate the moles of
 .
.
The balanced reaction is,
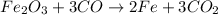
From the balanced reaction, we conclude that
As, 2 moles of iron obtained from 1 mole of
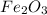
So, 0.276 moles of iron obtained from
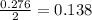 mole of
mole of
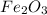
Now we have to calculate the mass of
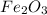
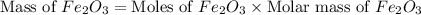
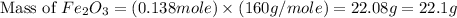
Therefore, the amount of
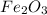 required are, 22.1 grams.
required are, 22.1 grams.