Answer: Option (B) is the correct answer.
Step-by-step explanation:
Molar mass is defined as the sum of masses of all the atoms present in a compound.
For example, atomic mass of barium is 137.32 g/mol and atomic mass of bromine is 79.90 g/mol.
Therefore, molar mass of
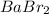 will be as follows.
will be as follows.
Molar mass = atomic mass of Ba +
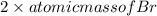
= 137.32 g/mol +
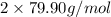
= 297.12 g/mol
Hence, we can conclude that molar mass of [tex]BaBr_{2}[tex] is 297.12 g/mol.