As we know that mass of the iron will remain same in solid state as well as in molten state as per mass conservation theory
Now we can say
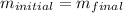
since mass is the product of volume and density so we can say
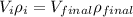
now from above equation we have
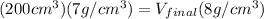
now by solving above equation we have
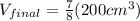
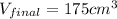
so final volume after it get solidify will become 175 cm^3