Answer:
X = 1,143 liters
Explanation:
To solve this problem let's pose the following equation:
(Amount of peroxide added + Amount initial of Solution)*(% of desired peroxide/100) = (amount of initial peroxide + amount of peroxide added).
* The added amount is unknown and we will call it X
* The percentage of peroxide desired is 16%
* The amount of initial peroxide is calculated as follows:
(%peroxide/ 100)(amount of initial substance)
= 0.08(12)
Finally the equation is:
(X + 12)(0.16) = 0.08(12) + X
0.16X -X = 0.96 - 1.92
-0.84X = -0.96
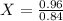
X = 1,143