Answer: A. A compound
Step-by-step explanation:
Compound is a pure substance which is made from atoms of different elements combined together in a fixed ratio by mass.It can be decomposed into simpler constituents using chemical reactions. Example: sodium hydroxide
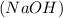
Element is a pure substance which is composed of atoms of similar elements.It can not be decomposed into simpler constituents using chemical reactions.Example: Copper
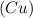
Heterogeneous mixture : It is a mixture that has non-uniform composition throughout the solution and the particle size or shapes are also different. There is a physical boundary between the dispersed phase and dispersion medium. Example: Air
Homogeneous mixture : It is a mixture that has uniform composition throughout the solution and the particle size or shapes are not different. There is no physical boundary between the dispersed phase and dispersion medium. Example: salt water