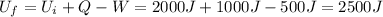Answer:
2500 J
Step-by-step explanation:
Let's solve the problem using the first law of thermodynamics, which states that
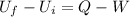
where
Uf is the final internal energy
Ui is the initial internal energy
Q is the heat absorbed by the system
W is the work done by the system
In this problem, we have:
Ui = 2000 J is the initial internal energy
Q = 1000 J is the heat absorbed by the system
W = 500 J is the work done by the piston
If we substitute numbers into the equation, we find: