Answer: Measure the volume of that compound which 56.17 ml for 100 grams of that compound.
Step-by-step explanation:
Density is defined as mass present in unit volume of the substance .

Density of the new liquid compound = 1.78 g/ml
In future if we wanted to to 100 gram of this compound without measuring its mass on the balance. What we can do is we can calculate its volume which we will require in a reaction with the help of density.
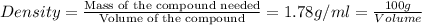
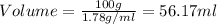
So, instead of measuring the mass of the compound we can directly measure the volume of that compound which 56.17 ml for 100 grams of that compound.