Answer:- 8.97 atm.
Solution:- Moles of each gas, volume of the vessel and temperature are given. So, we could easily use the partial pressure of each gas using ideal gas law equation.
PV = nRT
P is the pressure, V is the volume, n is the moles of the gas, R is universal gas constant and T is kelvin temperature.
The equation is rearranged for the pressure as:
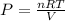
To calculate the partial pressure of fluorine we will use its moles.
n = 2.0 mol
V = 5.0 L
T = 273 K
R = 0.0821
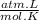
P = ?
Let's plug in the values and calculate the partial pressure of fluorine.
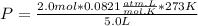
P = 8.97 atm
So, the partial pressure of fluorine gas is 8.97 atm.