Answer: Bowl B has the highest vapor pressure amongst the given bowls.
Step-by-step explanation:
Vapor pressure is defined as the pressure exerted by the vapor to its condensed state when both the states are in equilibrium at a given temperature. It is directly related to the temperature of the system.
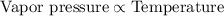
With increase in temperature, the kinetic energy of the particles increases and hence, will exert more pressure on the condensed state and higher will be the vapor pressure.
Hence, from the bowls given, the bowl which has high temperature will have the high vapor pressure.
Thus, bowl B has the highest vapor pressure amongst the given bowls.