Answer:-
 molecules.
molecules.
Solution:- The grams of tetrabromomethane are given and it asks to calculate the number of molecules.
It is a two step unit conversion problem. In the first step, grams are converted to moles on dividing the grams by molar mass.
In second step, the moles are converted to molecules on multiplying by Avogadro number.
Molar mass of
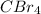 = 12+4(79.9) = 331.6 g per mol
= 12+4(79.9) = 331.6 g per mol
let's make the set up using dimensional analysis:
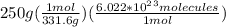
=
 molecules
molecules
So, there will be
 molecules in 250 grams of
molecules in 250 grams of
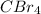 .
.