Answer:
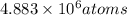 of strontium would be laid side by side to span a given distance.
of strontium would be laid side by side to span a given distance.
Step-by-step explanation:
Radius of strontium atom ,r= 215 pm =
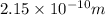
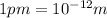
Diameter of the strontium atom = d = 2r:
d =
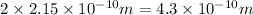 [/tex]
[/tex]
let the number of strontium atoms be x
Length of the chain of strontium atoms laid side by side = 2.10 mm
1 mm = 0.001 m
=2.10 mm = 0.001 × 2.10 m =
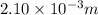
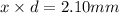
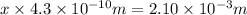
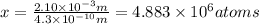
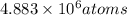 of strontium would be laid side by side to span a given distance.
of strontium would be laid side by side to span a given distance.