Answer: B. It is an ether because it is unable to to form a hydrogen bond, so it is less soluble than water
Step-by-step explanation: Alcohols
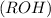 are more soluble in water as they can form hydrogen bonding with water whereas ethers
are more soluble in water as they can form hydrogen bonding with water whereas ethers
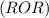 are less soluble as they do not form hydrogen bonds with water.
are less soluble as they do not form hydrogen bonds with water.
For formation of hydrogen bond, the electronegative atom (F, Oand N) must be attached to hydrogen and in ethers (ROR), there is no hydrogen directly attached to electronegative oxygen atom, thus are less soluble in water.