Answer: 155.134 grams of sulfuric acid is needed.
Step-by-step explanation:
To calculate the moles, we use the following equation:
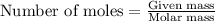
Moles of water:
Given mass of water = 57 grams
Molar mass of water = 18 g/mol
Putting values in above equation, we get:
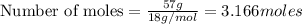
For the given chemical reaction, the equation follows:
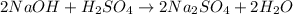
By Stoichiometry of the reaction:
2 moles of water are produced by 1 mole of sulfuric acid
So, 3.166 moles of water will produced by =
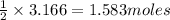 of sulfuric acid.
of sulfuric acid.
Now, to calculate the mass of sulfuric acid, we use the moles equation:
Molar mass of sulfuric acid = 98 g/mol
Putting values in above equation, we get:
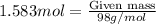
Mass of sulfuric acid = 155.134 grams
Hence, 155.134 grams of sulfuric acid is needed.