Answer: Option (d) is the correct answer.
Step-by-step explanation:
An oxidizing agent is a specie which accepts electrons and gets reduced in a chemical reaction.
In the given reaction,
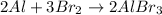
Oxidation state of Al changes from 0 to +3, therefore, it is reducing agent. Whereas oxidation state of Br is changing from -2 to -3 this means Br is gaining electrons, therefore, it is an oxidizing agent.
Thus, we can conclude that the statement
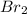 is the oxidizing agent because its oxidation number decreases.
is the oxidizing agent because its oxidation number decreases.