Answer:
The total quantity of the acid in the solution is 72.2 milliliters.
Explanation:
It is given that the solution contains acid and wat.
In the solution 18.6% is acid and remaining is wat.
The total quantity of the solution is 388 milliliters. So, the total quantity of the acid in the solution is 18.6% of 388.
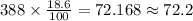
Therefore the total quantity of the acid in the solution is 72.2 milliliters.