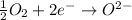Answer: It loses electrons to another element.
Explanation:- Oxidation is the process in which an element loses electrons and there is an increase in the oxidation state. On losing electrons it combines with a electronegative element such as oxygen, sulphur or nitrogen etc.
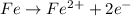
Reduction is the process in which an element gains electrons and there is a decrease in the oxidation state.