Answer:
An old bone contains 80% of its original carbon-14 in 1844.6479 years
Explanation:
We know that
half life time of C-14 is 5730 years
so, h=5730
now, we can use formula
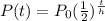
we can plug back h
and we get
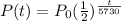
An old bone contains 80% of its original carbon-14
so,
P(t)=0.80Po
we can plug it and then we solve for t
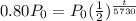
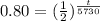
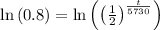
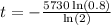
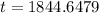
So,
An old bone contains 80% of its original carbon-14 in 1844.6479 years