Step-by-step explanation:
1) At STP, 1 mol of gas occupies 22.4 L of volume.
1 L of volume will be occupied by :
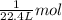
Then, 500 L of volume will be occupied by:
/(22.4 L)* 500L =22.32 mol\approx 22 mol](https://img.qammunity.org/2020/formulas/chemistry/middle-school/lo8xcfi45str7zu9rmrl7k5x6kskdhvnwl.png)
There will be 22 moles in 500 L of helium gas at STP.
2) Molecular mass of barium chromate= 253.37 g/mol
Atomic mass of chromium= 52 g/mol
Percentage of an element in a compound:
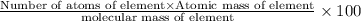
Percentage of chromium:
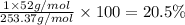
20.5% is the percentage of chromium in the given molecular formula.
3)The empirical formula of a substance that is 53.5% C, 15.5% H, 31.1% N by weight.
In 100 g of substance, mass of carbon = 53.5 g
Moles of carbon =
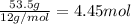
In 100 g of substance, mass of hydrogen= 15.5 g
Moles of carbon =
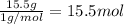
In 100 g of substance, mass of nitrogen= 31.1 g
Moles of carbon =
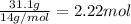
For empirical formula, divide smallest number of moles of an element from all the constituting moles of the elements.
Carbon =
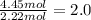
Hydrogen =
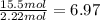
Nitrogen =
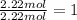
The empirical formula is
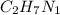 .
.
4) Combination reaction is a type of a chemical reaction in which two or more reactant reacts together to give single product.
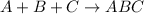
Oxide of metals are basic in nature and when they dissolved in water they form basic solution of their respective hydroxide.So, barium hydroxide is made by dissolving barium oxide(BaO) in water.
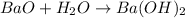
5)Replacement reaction is defined as chemical reaction in which an element displaces another element from its single compound.

Since, there are two reactants , out of which 1 is reactant compound. Both the reactants reacts to give two products.