Answer: The number of moles in 200 milliliters =10
The weight of 10 moles of glucose= 1800 grams =1.8 kg
Explanation:
Given: The number of moles in 100 milliliters =5
⇒ The number of moles in 1 milliliters =
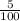
⇒The number of moles in 200 milliliters =
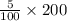
⇒The number of moles in 200 milliliters =10
Also, each mole of glucose weighs 180 grams .
Now, the weight of 10 moles of glucose=
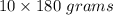
⇒ The weight of 10 moles of glucose= 1800 grams =1.8 kg