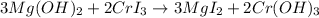Step-by-step explanation:
Magnesium is more reactive then chromium. So, when magnesium hydroxide reacts with chromium (lll) iodide then magnesium displaces chromium.
When magnesium hydroxide and chromium (lll) iodide are mixed together then it results in the formation of magnesium iodide and chromium hydroxide.
The chemical reaction equation is as follows.