Answer: A.) Removing a few marbles from the petri dish and stirring the rest around as energy is added
B) The high temperature makes the gas molecules spread apart according to Charles's law because this law describes how a gas will behave at constant pressure.
Explanation: The phase transition from solid to liquid involves the use of energy to make the molecules present in solid to break the inter molecular forces and to start moving away from each other as in liquid. The molecules in solid are closely packed whereas in liquids they are loosely packed. Thus less number of molecules are present per unit volume in a liquid. Thus the marbles have to be removed to show less density and the energy has to supplied. Removing all but two marbles from the petri dish and shaking them vigorously as energy is added will give us a more disorderd state called gas in which the molecules are very far apart and the density is least.
B) According to Boyle's law the pressure is inversely proportional to the volume of the gas at constant temperature and constant number of moles.
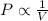 (At constant temperature and number of moles)
(At constant temperature and number of moles)
According to Charle's law the volume is directly proportional to the temperature of the gas at constant pressure and constant number of moles.
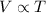 (At constant pressure and number of moles)
(At constant pressure and number of moles)
Thus as temperature of the gas increases , the volume also increases, and the density decreases. the gas becomes lighter and thus rises up.