Answer : The correct option is, A single product is formed.
Explanation :
- Synthesis reaction : It is defined as the chemical reaction in which the multiple substances or the reactants combines then it forms a single product.
The synthesis reaction is represented as,
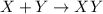
For example : When the hydrogen gas react with oxygen gas then its forms water as a single product.
The balanced chemical reaction will be :
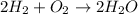
- Decomposition reaction : It is defined as a type of reaction in which a single larger compound decomposes to give two or more or multiple smaller molecules as a product.
The general representation of decomposition reaction is :
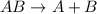
For example : When nitrogen dioxide decomposes then it gives oxygen gas and nitrogen gas as a products.
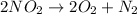
Hence, the true statements for a synthesis reaction is, a single product is formed.