Answer:
(1) Z = 82, M = 208; (2) Z = 82, M = 208
Explanation:
(1) Decay of Th-232
The unbalanced nuclear equation is
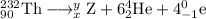
The main point to remember in balancing nuclear equations is that the sums of the superscripts and the subscripts must be the same on each side of the equation.
Then
90 = x + 6×2 + 4(-1), so x = 90 – 12 + 4 = 82
232 = y + 6×4 + 4×0, so y = 232 – 24 = 208
Z = 82, M = 208
(2) Decay of U-238
The unbalanced nuclear equation is
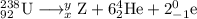
Then
92 = x + 6×2 + 2(-1), so x = 92 – 12 + 2 = 82
238 = y + 6×4 +4×0, so y = 238 – 24 = 214
Z = 82, M = 214