Answer : The volume of the gas is, 8.24018 L
Solution : Given,
Moles of gas = 2 moles
Pressure of gas = 1 atm
Temperature of gas = 50 K
Molar volume correction factor, b = 0.01709 L/mole
Gas constant, R = 0.08206 L atm/mole K
Formula used for real gas :
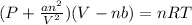
where,
P = pressure of gas
V = volume of gas
T = temperature of gas
R= gas constant
n = number of moles of gas
b = molar volume correction factor
a = pressure correction factor
The value of 'a' is zero
Now put all the given values in this formula, we get the volume of gas.
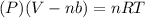
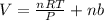
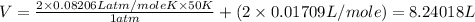
Therefore, the volume of the gas is, 8.24018 L