Answer: Elements and Compounds
Step-by-step explanation:
Mixture is a substance which has two or more components which do not combine chemically and do not have any fixed ratio in which they are present. Example: Air
Element is a pure substance which is composed of atoms of similar elements. It can not be decomposed into simpler constituents using chemical reactions.Example: iron
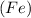
Solution is a homogeneous mixture of a solute and a solvent. Example: sugar dissolved in water
Compound is a pure substance which is made from atoms of different elements combined together in a fixed ratio by mass.It can be decomposed into simpler constituents using chemical reactions. Example: carbon dioxide

Thus Elements and Compounds are pure substances.