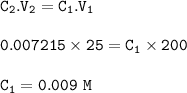The concentration of iron used in the titration : 0.009 M
Further explanation
Given
Reaction
Cr₂O₇²⁻ + 6Fe²⁺ + 14H⁺ ⇒ 2Cr³⁺ + 6Fe³⁺ + 7H₂O
0.0093 mol/L potassium dichromate
200 cm³ of dilute acid, 25cm³ was used in the titration.
Required
the concentration of iron
Solution
Titration formula
C₁V₁n₁=C₂V₂n₂⇒ From equation : n₁=6n₂(1=Cr₂O₇, 2=Fe)
titration average : 33+32.05+32.15+32.1 / 4 = 32.325 cm³(ml)
25 cm³ of iron solution used in titration :
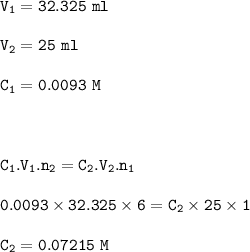
Dilution(25 ml from 200 ml iron solution)