Answer: Approximately 1.9 atm
============================================
Work Shown:
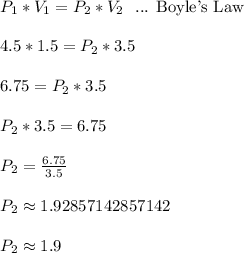
If the volume is 3.5 liters, then the pressure is approximately 1.9 atm.
Note the increase in volume leads to the reduction of pressure, and vice versa. The two variables have an inverse relationship.
-----------
As a check,
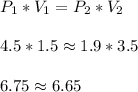
We don't get the exact thing on both sides, but the two sides are close enough. We have rounding error due to P2 being not exact.
A more accurate check could be
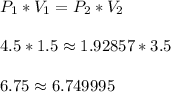
which has the two sides much closer to one another. This helps us verify the answer.