Answer:
183 K
General Formulas and Concepts:
Chemistry - Gas Laws
Combined Gas Law: PV = nRT
- P is pressure
- V is volume (in Liters)
- n is amount of moles
- R is gas constant -
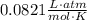
- T is temperature (in Kelvins)
Step-by-step explanation:
Step 1: Define
10.0 L
3.00 mol H₂
4.50 atm
Step 2: Find Temperature
- Substitute [CGL]:
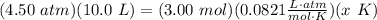
- Isolate temperature x:
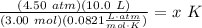
- Rewrite:
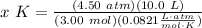
- Evaluate:
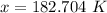
Step 3: Check
Round to the nearest whole number.
182.704 K ≈ 183 K