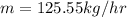Answer:
The appropriate response will be "125.55 kg/hr".
Step-by-step explanation:
The given values are:
Rate of heat exchanger,
m = 1000 kg/h
Inlet temperature,
T = 30°C
Specific heat,
 = 4 kJ/(kg°C)
= 4 kJ/(kg°C)
Now,
The heat taken by juice from steam will be:
⇒
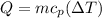
On substituting the values, we get
⇒
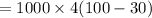
⇒
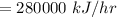
The heat given by the steam to juice will be:
⇒
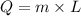
On substituting the values, we get
⇒
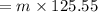
On equating both the heat values, we get
⇒
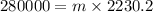
⇒
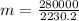
⇒