Answer:
a. The inlet temperature is approximately 305.232 K
b. The inlet pressure is approximately 452.0108 kPa
c. The area ratio between the inlet and exit is approximately 2.2509
Step-by-step explanation:
a. From the energy equation related to the question, we have;
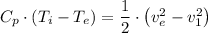
Where;
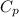 = The specific heat capacity for helium = 5.913 kJ/(kg·K)
= The specific heat capacity for helium = 5.913 kJ/(kg·K)
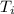 = The inlet temperature
= The inlet temperature
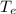 = The exit temperature = 300 K
= The exit temperature = 300 K
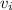 = The inlet velocity = 25 m/s
= The inlet velocity = 25 m/s
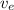 = The exit velocity = 250 m/s
= The exit velocity = 250 m/s
Therefore, we have;
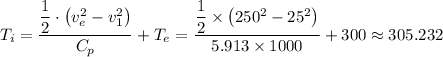
The inlet temperature =
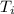 ≈ 305.232 K
≈ 305.232 K
b. From the following equation for the critical pressure, for helium, we have;
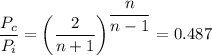
Where;
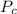 = The critical pressure = 2.26 atm for helim
= The critical pressure = 2.26 atm for helim
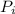 = The inlet pressure
= The inlet pressure
n = The polytropic constant
We have;
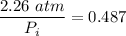
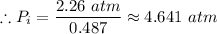
The inlet pressure,
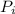 ≈ 4.641 atm ≈ 452.0108 kPa
≈ 4.641 atm ≈ 452.0108 kPa
c. The inlet to exit pressure ratio is given as follows;
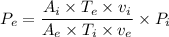
Therefore, we have;
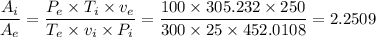
The area ratio between the inlet and exit,
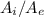 ≈ 2.2509.
≈ 2.2509.