Answer:
92.93378 kg
Explanation:
Given that:
Volume of Batch = 93 L
= 93 × 1000 (since 1 L = 1000 mL)
= 93000 mL
Specific gravity =
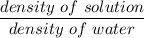
where;
density of water = 1 g/ml
specific gravity = 1.01 g/mL
Density of solution = (1.01 × 1) g/mL
Density of solution = 1.01 g/mL
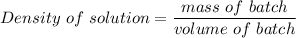
mass of batch = 1..01 g/mL × 93000 mL
mass of batch = 93930 g
Now, the mass of additional water = mass of (batch - Ropivaciance - sodium chloride)
= 93930 g - 196.42 g - 799.8 g
= 92933.78 g
Since 1kg = 1000 g
The weight of the water to be added to the QS solution is:
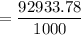
= 92.93378 kg