Additional information
Relative atomic mass(Ar) : A=7, G=16
The empirical formula : A₂G
Further explanation
Given
3.5g of element A
4.0g of element G
Required
the empirical formula for this compound
Solution
The empirical formula is the smallest comparison of atoms of compound forming elements.
The empirical formula also shows the simplest mole ratio of the constituent elements of the compound
mol of element A :
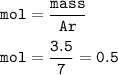
mol of element G :
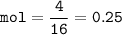
mol ratio A : G = 0.5 : 0.25 = 2 : 1