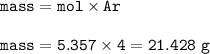The mass of He gas = 21.428 g
Further explanation
Given
120 liters of He gas
Required
the mass in grams
Solution
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
Mol for 120 L :
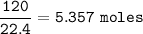
Mass of He gas(MW=4 g/mol) :