Answer:
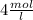
Step-by-step explanation:
From the question, we have been asked to find the molarity of FeCl2 having a volume of 450 mL,
We have been provided with 225 g which is proportional to 1.8 moles.
We know that molarity of any solution should be in mol/L.
1 mole contained in 1 L means it has a molarity of 1 mol/L
Let's convert 450 mL to Litres which is,
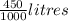
= 0.450 L
Thus,
1 mole is contained in 1L
x moles are contained in 0.450 L
Hence,
x mole/molarity = {1 mole x 1 L}/{0.450 L}
= 4 mol/L
Therefore 4 mol/L is the molar concentration.