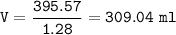The volume = 309.04 ml
Further explanation
Given
Specific gravity=-1.28 and strength =24.7% by mass of H₂SO₄
mass 125g of ZnC0₃
Required
The volume of H₂SO₄ Solution
Solution
Reaction
ZnCO₃ + H₂SO₄ → ZnSO₄ + CO₂ + H₂O
mol of ZnCO₃(MW=125.4 g/mol) :
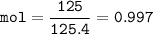
From equation, mol ZnCO₃ : H₂SO₄= 1 : 1 so mol H₂SO₄=0.997
mass of H₂SO₄ (MW=98 g/mol) :
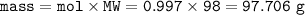
mass of solution :
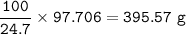
Volume of solution :(density of solution=1.28 g/ml for the reference substance is water(density=1 g/ml)