Step-by-step explanation:
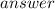
The overall cell potential can be calculated by using the equation E0cell=E0red−E0oxid. Step 2: Solve. Before adding the two reactions together, the number of electrons lost in the oxidation must equal the number of electrons gained in the reduction. The silver half-cell reaction must be multiplied by two.