Answer:
a. HCl.
b. 0.057 g.
c. 1.69 g.
d. 77 %.
Step-by-step explanation:
Hello!
In this case, since the reaction between magnesium and hydrochloric acid is:
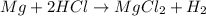
Whereas there is 1:2 mole ratio between them.
a) Here, we can identify the limiting reactant as that yielded the fewest moles of hydrogen gas product via the 1:1 and 2:1 mole ratios:
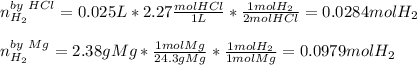
Thus, since hydrochloric yields fewer moles of hydrogen than magnesium, we realize it is the limiting reactant.
b) Here, we use the molar mass of gaseous hydrogen (2.02 g/mol) to compute the mass:
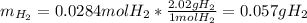
c) Here, we compute the mass of magnesium associated with the yielded 0.0248 moles of hydrogen:
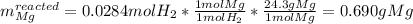
Thus, the mass of excess magnesium turns out:
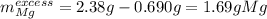
d) Finally, we compute the percent yield, considering 0.044 g is the actual yield and 0.057 g the theoretical yield:
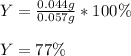
Best regards!