Answer:
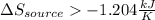
Step-by-step explanation:
Hello!
In this case, given the initial conditions, we first use the 10-% quality to compute the initial entropy:
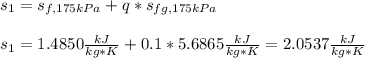
Now the entropy at the final state given the new 40-% quality:
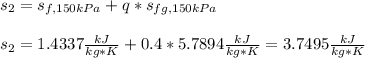
Next step is to compute the mass of steam given the specific volume of steam at 175 kPa and the 10% quality:

Then, we can write the entropy balance:
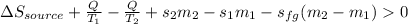
Whereas sfg stands for the entropy of the leaving steam to hold the pressure at 150 kPa and must be greater than 0; thus we plug in:
Which is such minimum entropy change of the heat-supplying source.
Best regards!