The mass of sodium hydrogen carbonate : 10.5 g
Further explanation
Given
1.5 dm' of CO₂
1 mol gas= 24 L at RTP(25 °C, 1 atm)
Required
the mass of sodium hydrogen carbonate
Solution
Decomposition reaction of Sodium hydrogen carbonate :
2 NaHCO₃ (s) ⇒ Na₂ CO₃ (s) + H₂ O(g) + CO₂ (g)
mol CO₂ :
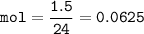
From the equation, mol ratio of NaHCO₃ : CO₂ (g) = 2 : 1, so mol NaHCO₃ :
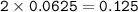
Mass NaHCO₃(MW=23+1+12+3.16=84 g/mol) :
