Given :
15 gram of ice is kept at 0°C.
To Find :
How much heat is required to melt 15 grams of ice at 0°C.
Solution :
We know, specific heat of Ice, L = 334 J/g.
Heat required to melt m gram of ice to water at 0°C is :
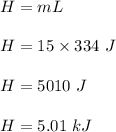
Therefore, heat is required to melt 15 grams of ice at 0°C is 5.01 kJ.