First you need to write out the original equation.
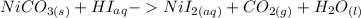
It needs to be balanced, though, so after balancing it should look like this:
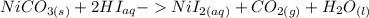
The ionic equation would look like this. (Only aqueous solutions can be split up).
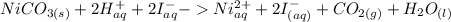
The net ionic equation only contains the products that are not aqueous. Therefore, the final net ionic equation should be:
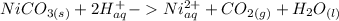
So your answer is C.