Answer:
E
Step-by-step explanation:
The vander waals equation
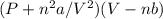 =nRT
=nRT
The factor n^2a/V^2 accounts for the attractive forces between the gases molecules whereas nb accounts for the decrease in volume occupied by the gas. Therefore, correct option would be E. None of the above have BOTH of the two factors accurately stated