The sign's glass absorbed 25466.7 J
Further explanation
Given
The temperature of glass : 23.5 °C to 65.5 °C
mass = 905 g
the specific heat capacity = 0.67 J/g °C
Required
Heat absorbed
Solution
Heat absorbed by sign's glass can be formulated :
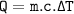
ΔT=65.5 - 23.5 = 42
